Antibody Purification: Methods, Applications and Key Technologies
Purification is critical to isolating antibodies in a pure, functional form suitable for downstream applications.
Complete the form below to unlock access to ALL audio articles.
Antibodies are essential proteins produced by the immune system that recognize and bind to specific targets, known as antigens. Their high specificity and affinity make antibodies invaluable tools in life sciences research, diagnostics and increasingly, as therapeutic agents for diseases such as cancer, autoimmune disorders and infectious diseases. However, antibodies produced naturally or recombinantly are usually part of complex mixtures containing other proteins, cell debris and impurities. This makes antibody purification a critical step to isolate antibodies in a pure, functional form suitable for downstream applications.
This article provides an overview of antibody purification methods, highlighting key techniques such as affinity chromatography (including protein A and protein G), ion exchange chromatography and size exclusion chromatography (SEC). We also explore purification challenges specific to antibody classes like immunoglobulin G (IgG) and immunoglobulin M (IgM), and briefly touch on real-world applications to illustrate why antibody purity is so important in research and drug discovery.
What is antibody purification?
Monoclonal vs polyclonal antibody purification
- Monoclonal antibodies
- Polyclonal antibodies
Overview of antibody purification methods
- Ammonium sulfate precipitation
- Affinity chromatography
- Protein A purification
- Protein G purification
- Ion exchange chromatography
- Size exclusion chromatography
Purifying different antibody isotypes
- IgG purification
- IgM purification
What is antibody purification?
Antibody purification is the process of isolating antibodies from a mixture that contains other proteins, cellular components and impurities. Purification is critical because raw antibody preparations often contain contaminants that can interfere with experiments or cause adverse reactions if used clinically. For example, impurities can cause non-specific binding in assays or provoke immune responses in patients receiving antibody therapies. Some products produced by antibody sources, such as Chinese hamster ovary (CHO) cells and human embryonic kidney (HEK) tissue cells, secrete immunogenic proteins not suitable for humans.
The purification process balances two key factors: purity (how free the antibody is from other substances) and yield (how much antibody is recovered). High purity is essential for applications demanding precision, such as structural studies or drug development, but achieving it often means some loss of yield. Therefore, the choice of purification method depends on the intended use of the antibody and the source material.
Purified antibodies find widespread use in life sciences, including in immunoassays like the enzyme-linked immunosorbent assay (ELISA) and western blotting, flow cytometry, immunohistochemistry and as therapeutics that target cancer cells or modulate immune responses.
Monoclonal vs polyclonal antibody purification
Antibodies can be broadly categorized into two types: monoclonal and polyclonal. Understanding the differences between these is essential because they influence the approach and challenges in antibody purification.
Monoclonal antibodies
Monoclonal antibodies (mAbs) are produced by identical immune cells cloned from a single parent cell. This results in antibodies that are uniform in structure and specificity, binding to a single epitope on an antigen. Monoclonal antibodies are widely used in therapeutic treatments, diagnostics and highly specific research applications due to their reproducibility and precision. In research, monoclonal antibodies can be used to identify cells using surface receptor-specific antibodies in flow cytometry. As a therapeutic, monoclonal antibodies can be used to target overexpression of certain surface proteins on tumors for immune cell stimulation, such as against the HER2 protein on HER2-positive breast cancer.
Because of their uniformity, monoclonal antibody purification methods are often highly optimized to yield pure, active antibodies. Hybridoma cell culture supernatants or recombinant expression systems are common sources. Purification techniques for mAbs focus on maintaining antibody integrity and functionality, which is critical for therapeutic use where regulatory standards demand high purity.
Polyclonal antibodies
Polyclonal antibodies are produced by multiple different B cell clones in response to an antigen, meaning they recognize multiple epitopes with structural diversity. These antibodies are usually harvested from animal serum and contain a heterogeneous mixture of immunoglobulins, which may include IgG, IgM and other isotypes. Intravenous immunoglobulin is an example of a polyclonal antibody therapy that utilizes pooled IgG antibodies from donors and has long been used to treat several autoimmune diseases.1
Purifying polyclonal antibodies is often more complex due to this diversity. The presence of multiple antibody types and other serum proteins means that purification methods must be carefully selected and combined to isolate the target antibodies while removing unwanted proteins. This can involve more extensive fractionation and multiple purification steps.
The choice of purification method depends heavily on whether the antibody is monoclonal or polyclonal (Table 1), as well as the downstream applications and purity requirements.
Table 1. Key differences in purification approaches for monoclonal vs polyclonal antibodies.
| Monoclonal Antibodies | Polyclonal Antibodies | |
| Source | Hybridoma cultures, recombinant systems such as CHO and HEK cells | Animal serum (e.g., rabbit, goat) |
| Homogeneity | Uniform antibody paratope | Mixture of antibody paratopes |
| Purification complexity | Generally simpler, more standardized methods | More complex, may require multiple steps |
| Applications | Therapeutics, diagnostics and precise research | Research, less specific assays |
Overview of antibody purification methods
Antibody purification employs a variety of techniques, each with its advantages and limitations (Table 2). Selecting the appropriate method depends on factors such as the antibody type, source material, required purity and scale of purification. Common purification methods include precipitation and chromatographic techniques like affinity chromatography, ion exchange chromatography and SEC. Ultrafiltration and diafiltration are also often used for concentration and buffer exchange.
Affinity chromatography is often considered the gold standard for routine bioprocessing and bench-scale production, given that protein A and protein G are highly versatile for IgGs. Other methods are often only used if affinity-based binding is not available for that subclass. Ion exchange and SEC are commonly used as polishing steps to enhance purity and remove aggregates or remaining impurities.
Table 2. Advantages, limitations and applications of some of the common antibody purification techniques.
| Method | Principle | Advantages | Limitations | Applications |
| Ammonium sulfate precipitation | Salting out proteins by reducing solubility | Cost-effective, simple, good for bulk separation | Low specificity, requires further purification | Crude antibody mixtures |
| Affinity chromatography | Specific binding between antibody and ligand | High selectivity and purity | Resin cost, sometimes harsh elution conditions | IgG, monoclonal antibodies |
| Ion exchange chromatography | Separation based on charge differences | Good polishing step, scalable | Requires careful pH control | Polyclonal antibodies, polishing, removal of charge variants |
| Size exclusion chromatography | Separation by size | Gentle, good for removing aggregates | Low capacity, time-consuming | Final polishing, aggregation analysis |
Ammonium sulfate precipitation
Ammonium sulfate precipitation is a cost-effective method used to concentrate and partially purify antibodies from complex mixtures such as serum or cell culture supernatants. This technique relies on the principle of “salting out,” where increasing the concentration of ammonium sulfate reduces the solubility of proteins, causing them to precipitate out of solution.
As ammonium sulfate is added to the antibody-containing solution, water molecules become increasingly associated with the salt ions, reducing the amount of free water available to solubilize proteins. This decreases protein solubility, causing them to aggregate and precipitate. By carefully controlling the concentration of ammonium sulfate, specific proteins, including antibodies, can be selectively precipitated while leaving other impurities in solution.
Advantages
- Cost-effective and simple: Ammonium sulfate is inexpensive, and the procedure does not require specialized equipment.
- Scalable: Suitable for processing large volumes of crude antibody mixtures.
- Gentle: Maintains antibody activity and structure during precipitation.
Limitations
- Low specificity: This method separates proteins based on solubility, not affinity or charge, so it often requires additional purification steps to achieve high purity.
- Labor intensive: Requires centrifugation or filtration to recover precipitated proteins and removal of residual salt through dialysis or diafiltration.
Ammonium sulfate precipitation is often used as an initial purification or concentration step before more selective methods like affinity chromatography. It helps reduce sample volume and remove bulk contaminants, streamlining subsequent purification stages. The method is typically only used in low producers in high-volume supernatant and has been largely replaced by advanced chromatography techniques.
Affinity chromatography
Affinity chromatography is one of the most widely used and effective methods for antibody purification. It leverages the highly specific interaction between an antibody and a ligand attached to a chromatography resin (Figure 1). This selectivity allows for efficient isolation of antibodies from complex mixtures, often yielding high purity in a single step.
In affinity chromatography, the antibody-containing solution is passed through a column packed with resin coated with a ligand that specifically binds antibodies. The antibodies attach to the ligand while other proteins and impurities flow through. The bound antibodies are then eluted by changing conditions such as pH or ionic strength, disrupting the antibody-ligand interaction.
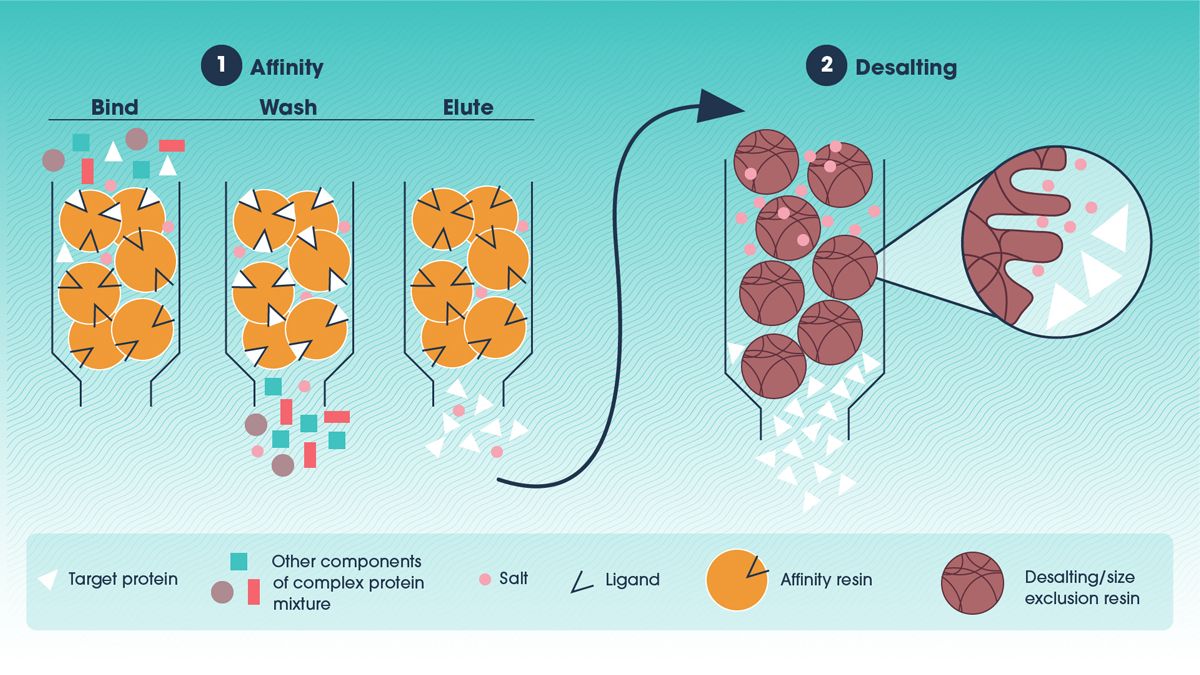
Figure 1. An example of a two-step antibody purification protocol utilizing affinity chromatography and desalting. Credit: Technology Networks.
This method is particularly popular for purifying IgG antibodies, which are commonly targeted in therapeutic and research applications.2 Two of the most common affinity-based methods utilize protein A and protein G.
Protein A purification
Protein A is a bacterial cell wall protein derived from Staphylococcus aureus that binds specifically to the Fc region of IgG antibodies, particularly human IgG subclasses 1, 2 and 4. Its high affinity and specificity make protein A chromatography a gold standard for purifying monoclonal IgG antibodies.3
- High binding capacity and specificity for IgG antibodies.
- Enables purification directly from complex sources like cell culture supernatants or serum.
- Produces antibodies with high purity and activity.
Limitations:
- Lower affinity for certain IgG subclasses (e.g., IgG3).
- Elution at low pH can sometimes affect stability in antibodies that are pH sensitive; however, a low pH is used as a method of viral inactivation in production settings.
Protein G purification
Protein G, derived from Streptococcus species, also binds to the Fc region of IgG but with a different binding profile compared to protein A. Protein G has a stronger affinity for some IgG subclasses, such as human IgG3 and mouse IgGs, making it useful when protein A is less effective.4 In addition, protein G can now be made recombinantly, which brings down the overall price of the method.
Advantages:
- Broader binding profile across species and IgG subclasses.
- Often used to purify mouse monoclonal antibodies or human IgG3.
Limitations:
- Slightly lower binding capacity than protein A in some cases.
- Similar to protein A, elution conditions can impact antibody stability.
Ion exchange chromatography
Ion exchange chromatography separates antibodies based on their charge properties. Antibodies, like other proteins, carry charged groups that can bind to oppositely charged groups on a resin. By adjusting the pH and ionic strength of the buffer, antibodies can be selectively bound to and eluted from the resin (Figure 2).
There are two main types of ion exchange chromatography:
- Cation exchange chromatography: Uses negatively charged resin to bind positively charged proteins.
- Anion exchange chromatography: Uses positively charged resin to bind negatively charged proteins.
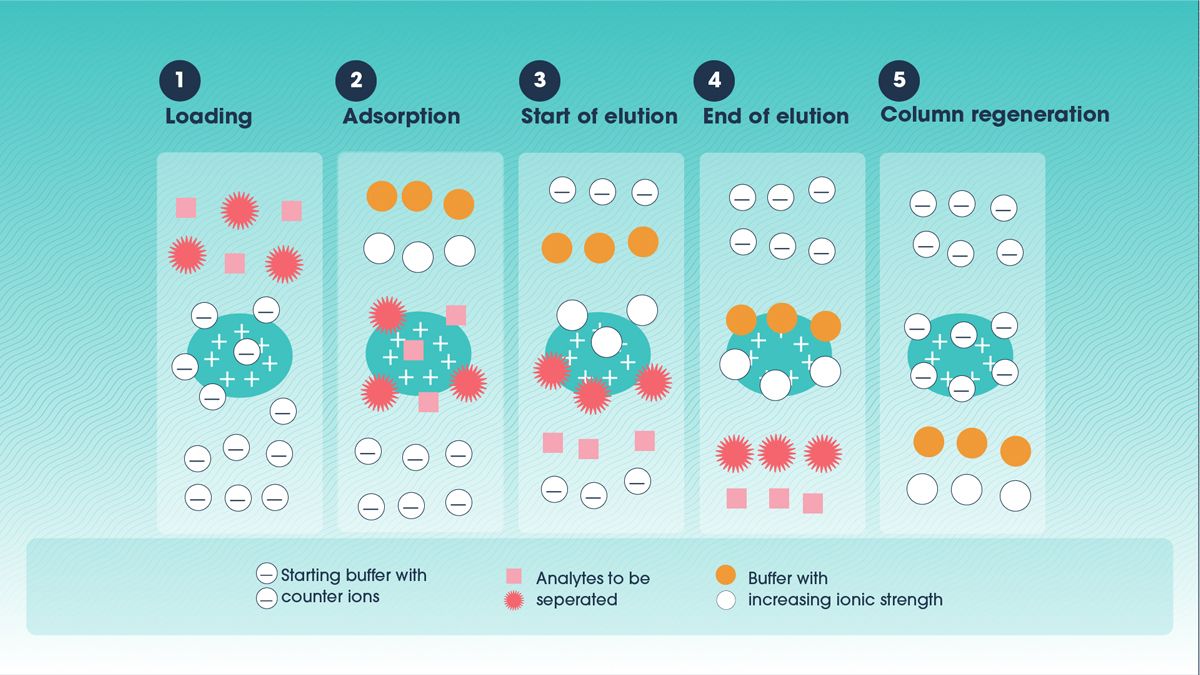
Figure 2. Schematic diagram showing analyte separation in ion exchange chromatography by increasing the ionic strength of the elution buffer. Credit: Technology Networks.
Ion exchange chromatography is often used as a polishing step following affinity chromatography to remove impurities such as host cell proteins, DNA or antibody aggregates.5 It can enhance purity and improve product quality by exploiting subtle charge differences.
Advantages:
- High capacity and scalability.
- Good for fine purification and removal of closely related contaminants.
Limitations:
- Requires careful control of pH and salt gradients.
- Does not typically remove closely associated proteins of the same charge (co-elution) and typically not suitable for removing aggregates.
Size exclusion chromatography
SEC separates proteins based on their size by passing the sample through a porous resin. Larger molecules elute first because they are excluded from the pores, while smaller molecules enter the pores and take longer to pass through the column.
It is typically used as a final polishing step in antibody purification to remove aggregates or fragments, ensuring a uniform, monomeric antibody preparation.6 It is gentle on antibodies, preserving their activity and structure.
Advantages:
- Non-destructive and gentle.
- Effective at removing aggregates and small impurities.
Limitations:
- Low throughput and capacity.
- Generally not suitable for large-scale primary purification.
Purifying different antibody isotypes
IgG purification
IgG is the most abundant antibody isotype in serum and the most commonly purified antibody for research and therapeutics. Its well-characterized structure and strong binding affinity for protein A and protein G make it ideal for affinity chromatography methods, which dominate IgG purification workflows.
IgM purification
IgM antibodies are pentameric, larger and structurally more complex than IgG. Several naturally occurring IgM antibodies have been explored as therapeutics, and engineered IgM antibodies with enhanced binding and/or additional functional properties are being evaluated in humans.7
Their size and tendency to aggregate make purification more challenging. Protein A and protein G have limited affinity for IgM, so alternative strategies like SEC or customized affinity ligands are often used. Polyethylene glycol precipitation can also be used, as well as SEC, since the pentameric form of IgM is close to 1,000 KDa.
Due to their complexity, IgM purifications typically require gentle handling and multiple steps to maintain activity and yield.
Applications of purified antibodies
Purified antibodies are essential in various fields:
- Therapeutics: Monoclonal antibodies are widely used as treatments for cancer, autoimmune diseases and infectious diseases, offering targeted therapy with reduced side effects.8, 9, 10
- Diagnostics: Purified antibodies serve as reagents in diagnostic assays to detect biomarkers or pathogens with high sensitivity and specificity.11
- Research: Antibodies are used in techniques such as western blotting, immunohistochemistry, ELISA, flow cytometry and immunoprecipitation to study protein expression, localization and interactions.
Best practices and emerging trends
Selecting the right antibody purification method depends on antibody type, source, intended use and scale. Key considerations include maintaining antibody integrity, maximizing yield and achieving the required purity.
Emerging trends in antibody purification include:
- Automation: Increasing use of automated chromatography systems to improve reproducibility and throughput.12
- Single-use technologies: Disposable columns and membranes reduce cross-contamination risks and cleaning requirements.
- Continuous purification: Novel flow-through systems enable constant processing, reducing time and cost.13
- AI and machine learning: Early applications in optimizing purification conditions and predicting resin performance.14
These advancements aim to streamline purification workflows, reduce costs and improve product quality in both research and biopharmaceutical manufacturing.
Antibody purification is a vital process in life sciences, ensuring antibodies are isolated in pure, active forms for research, diagnostics and therapeutic applications. Understanding the strengths and limitations of various purification methods, from ammonium sulfate precipitation to advanced chromatographic techniques, enables researchers to select the most suitable approach for their needs. As technology advances, new methods and automation promise to make antibody purification more efficient, scalable and accessible.
This content includes text that has been generated with the assistance of AI. Technology Networks' AI policy can be found here.